Functional interfaces for Nanotechnology
Research interests:
Energy-related applications
The reorganisation of liquid molecules at the surface of a solid is naturally driven by molecular interactions between both media. The equilibrium arrangement of the liquid molecules is driven by minimisation of the interface free energy. It is possible to externally shift this equilibrium by adding new contributions to the free energy, hence also changing the molecular organisation of the interface. This achieved, for example, in the case of electrowetting on dielectrics where an added electrical component to the free energy landscape shifts its minimum, leading to reorganisation of the interfacial liquid, inducing in turn a macroscopic change in wetting.
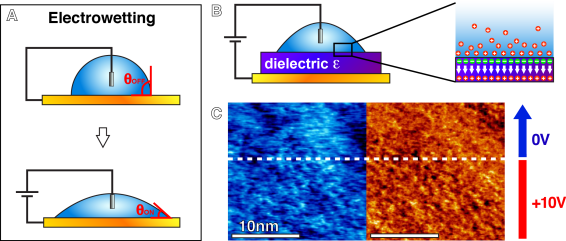
Here the effect of trans-interface electrical fields on the structure and dynamics of the interfacial liquid at the nanoscale is explored. The idea is to exploit metastable interfacial systems that can be switched between several states (molecular arrangements of the liquid). This can typically be achieved with liquid mixtures exhibiting dissimilar affinities for the solid.The long-term goal of this research line is to develop functional interfaces where the externally driven re-organisation of the interfacial liquid can be exploited to template molecular assemblies, laterally displace adsorbed molecules or guide lateral diffusion.Importantly, no chemical bonds are used, the functional interfaces are themselves self-assembled structures at equilibrium with the bulk liquid.
